SAKK trials publication rate reaches over 95%
Increased completion rate
76 of 261 (29%) trials were closed earlier than planned. The three main reasons were that the target number of patients could not be reached (28 trials), followed by stopping for futility (17), and in 8 trials it became earlier apparent that the intervention will provide substantial benefit (8 trials).
The findings are in line with other studies, highlighting that not reaching the planned number of patients is still the main reason for trial discontinuation. However, the rate of successful completion of SAKK trials increased over time reaching a level of about 75% for the last 15 years. One of the key factors may be continuous implementation of quality management during selection, set-up and conduct of SAKK trials.
High publication rate
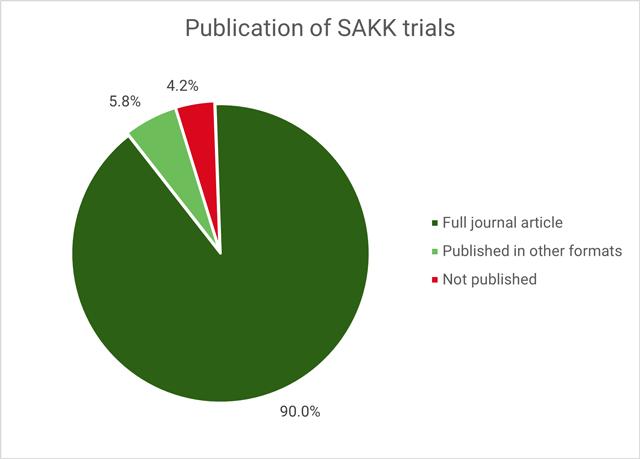
To investigate how often SAKK trials were published, 240 trials were included. 21 trials had to be excluded for this analysis, because follow-up was still ongoing or the trial was just recently closed within the last 12 months. 216 of 240 (90%) were published as full article in a peer-reviewed journal, 14 were published in other formats, leading to an overall publication rate of 96%.
Trials that were closed earlier were less likely to be published. However, even for these trials, the publication rate was around 80%, which is markedly higher than reported in the literature. One of the main reasons for this improvement could be the cooperative group setting and its continuous development of professionalization, for example the SAKK publication guidelines.
Conclusion: High values thanks to quality management
In summary, the authors found that successful completion of SAKK trials was high and importantly, increased over time. SAKK has continuously improved its quality management of trial conduct over time leading to increased successful trial completion and publication. Overall, over 95% of SAKK trials were published, 90% as full article in a peer-reviewed journal.
Authors
- Dr. Stefanie Hayoz SAKK
- Dr. med. Benjamin Kasenda University hospital Basel
- Annina Lea Schenker SAKK
- Dr. Christoph Kopp SAKK
- Dr. Sämi Schär SAKK
- Prof. Dr. med. Beat Thürlimann Cantonal hospital St. Gallen
- Prof. Dr. med. Roger von Moos Cantonal hospital Graubünden
- Prof. Dr. med. Miklos Pless Cantonal hospital Winterthur
Publication
Hayoz S, Kasenda B, Schenker AL, et al. Completion and publication of clinical trials in a cooperative group: a cohort study of trials of the Swiss Group for Clinical Cancer Research (SAKK). BMJ Open 2023;0:e068490. doi:10.1136/
https://dx.doi.org/10.1136/bmjopen-2022-068490
About the Swiss Group for Clinical Cancer Research (SAKK)
The Swiss Group for Clinical Cancer Research (SAKK) is a non-profit organization, which has been conducting patient-oriented clinical cancer trials since 1965. The regular members of SAKK are the main clinical-oncology sites at the cantonal and private hospitals and university hospitals. They work with other hospitals and physicians, and together form the SAKK network. The Competence Center of SAKK in Bern helps researching physicians develop and conduct multicenter and interdisciplinary trials independently of the pharmaceutical industry.